FDA Drug Recall NoticeFDA Drug Recall Notice
Fresenius Kabi Issues Voluntary Nationwide Recall of Two Lots of Fluorouracil Injection Due to the Potential for Glass Particulate When a company announces a recall, market withdrawal, or safety alert,

Fresenius Kabi Issues Voluntary Nationwide Recall of Two Lots of Fluorouracil Injection Due to the Potential for Glass Particulate When a company announces a recall, market withdrawal, or safety alert,
FIRST CATARACT SURGERY SINCE 1997 WICKENBURG, AZ – April 23, 2019, Wickenburg Community Hospital (WCH) expands
Flu & RSV remains a threat to the health & wellness of our community. In the past week there were 562 lab confirmed influenza cases with 41 lab confirmed RSV
RECOGNITION AS DISTINGUISHED DOCTOR OF INTERNAL MEDICINE WICKENBURG, AZ – April 15, 2019, Dr. Todd Kravetz, MD, FACP, Wickenburg Community Hospital Chief of Staff, Clinic Medical Director and Physician of
https://www.facebook.com/WickenburgCommunityHospital/videos/394337141298124/

WCH has admitted our first flu patient last week. Some areas of the country are already starting to see an increased number of cases. If that is the case, this

For Immediate Release: October 4, 2018 Media Contact: Melissa Blasius-Nuanez Mobile: 480-320-0592 Raw Beef Products Recalled People should discard or return recalled meat to avoid foodborne illness PHOENIX — The
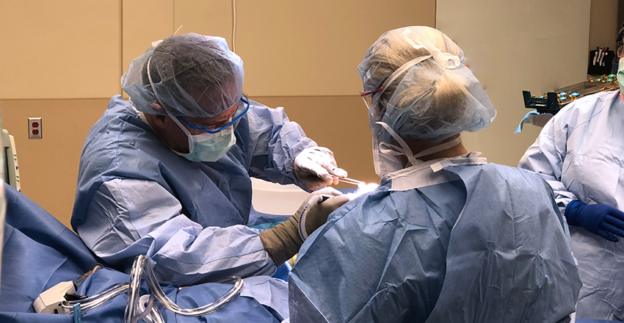
Two years have passed since the ribbon was officially cut on the Wickenburg Community Hospital Diagnostic and Surgical Center. Orthopedic surgeon Mitch Wagner, MD, who has been there from the

Do you suffer from diabetes? It can be challenging and, yes, even frustrating. Luckily science, medicine and even technology are making advances that make day to day activity much more

Written by: Roger Rose, RPh, Director of Pharmacy Services Did you know that there are various types of baldness, and the treatments differ? About half the men and women show